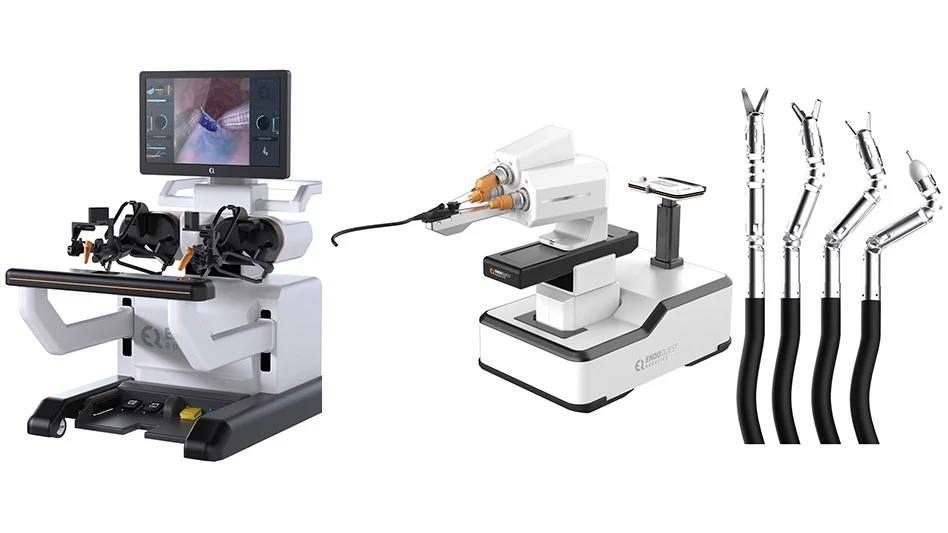
EndoQuest Robotics Secures Series D-2 Funding to Accelerate Breakthrough in Robotic Endoluminal Surgery
With backing from Crescent Enterprises and Dr. Fred Moll, EndoQuest advances its flexible endoluminal surgical platform through the pivotal PARADIGM trial, aiming for FDA clearance and transforming minimally invasive GI procedures with next-gen robotic technology.
Image Courtesy: Public Domain
EndoQuest Robotics, Inc., a pioneering leader in endoluminal robotic surgical technology, announced that it has successfully closed a Series D-2 financing round. This strategic funding will fuel the advancement of EndoQuest’s flexible endoluminal surgical platform, supporting the ongoing pivotal PARADIGM clinical trial and accelerating the pathway towards U.S. FDA clearance, as well as early feasibility studies in the upper GI tract and progression of advanced endoluminal visualization technologies in the Company’s portfolio. Funding for the round was co-led by Crescent Enterprises and Dr. Fred Moll.
Dr. Moll commented that "EndoQuest has developed a groundbreaking capability that could revolutionize how procedures are performed in robotic endoscopic surgery." A recognized pioneer in the field, Dr. Moll went on to say, "I observed the first clinical cases which were very impressive with regard to the novel capabilities of the system."
Neeraj Agrawal, Executive Director of Crescent Enterprises and Director at EndoQuest, said: “EndoQuest perfectly fits our investment thesis of targeting high-CAGR surgical robotics fields where robotic solutions can verticalize and capture value across the entire operating theater. We focus on companies that operate in surgical theaters where there is no presence from incumbents, and EndoQuest’s endoluminal platform exemplifies this. We are also highly encouraged by the strong interest from strategic partners, which further validates our confidence in EndoQuest’s vision and its potential to reshape surgical care.”
Eduardo Fonseca, EndoQuest Robotics Interim CEO, said: “Our team is laser-focused on delivering four key pillars of value as we advance our platform. These include stellar clinical data from our trials, an attractive pathway to FDA clearance, progression of the world’s most advanced endoluminal visualization systems, and the pioneering of new upper GI clinical procedures in parallel with our ongoing IDE trial. Through focused execution across these four pillars, we are working to bring the impact of our pioneering technology to patients and physicians worldwide."
Nicholas Drysdale, EndoQuest Robotics Chief Financial Officer, said: “Building on strong momentum and a rapidly expanding technology portfolio with high-impact clinical applications, we are positioned to deliver meaningful long-term returns for our investors. Across both our U.S. and Korea campuses, we remain committed to capital discipline and focused execution.”
Other investors in the round included Puma Venture Capital and The University of Texas Health Science Center at Houston (UTHealth Houston).
The PARADIGM Trial (Prospective Assessment of a Robotic Assisted Device in Gastrointestinal Medicine) is currently underway and recently achieved its first clinical milestone, where two colorectal endoscopic submucosal dissection (ESD) procedures were successfully completed by Dr. Eric Haas at HCA Houston Healthcare Medical Center using EndoQuest’s Endoluminal Surgical (ELS) System. These initial procedures – performed on complex colorectal lesions including a fibrotic lesion over 5 cm in size* – demonstrated the ELS System’s ability to reach and treat lesions that most likely would require more invasive surgery. The investigational device exemption (IDE)-approved PARADIGM study is slated to enroll 50 patients across five leading U.S. hospitals (Brigham and Women’s Hospital, Mayo Clinic, Cleveland Clinic, AdventHealth, and HCA Healthcare), and will evaluate the safety and effectiveness of the ELS System in the removal of colorectal lesions (Clinicaltrials.gov).
Upon successful completion of the trial, EndoQuest plans to seek market authorization for the ELS System in the United States.
* Lesion characteristics are reported for descriptive purposes only and are not indicative of clinical outcomes.