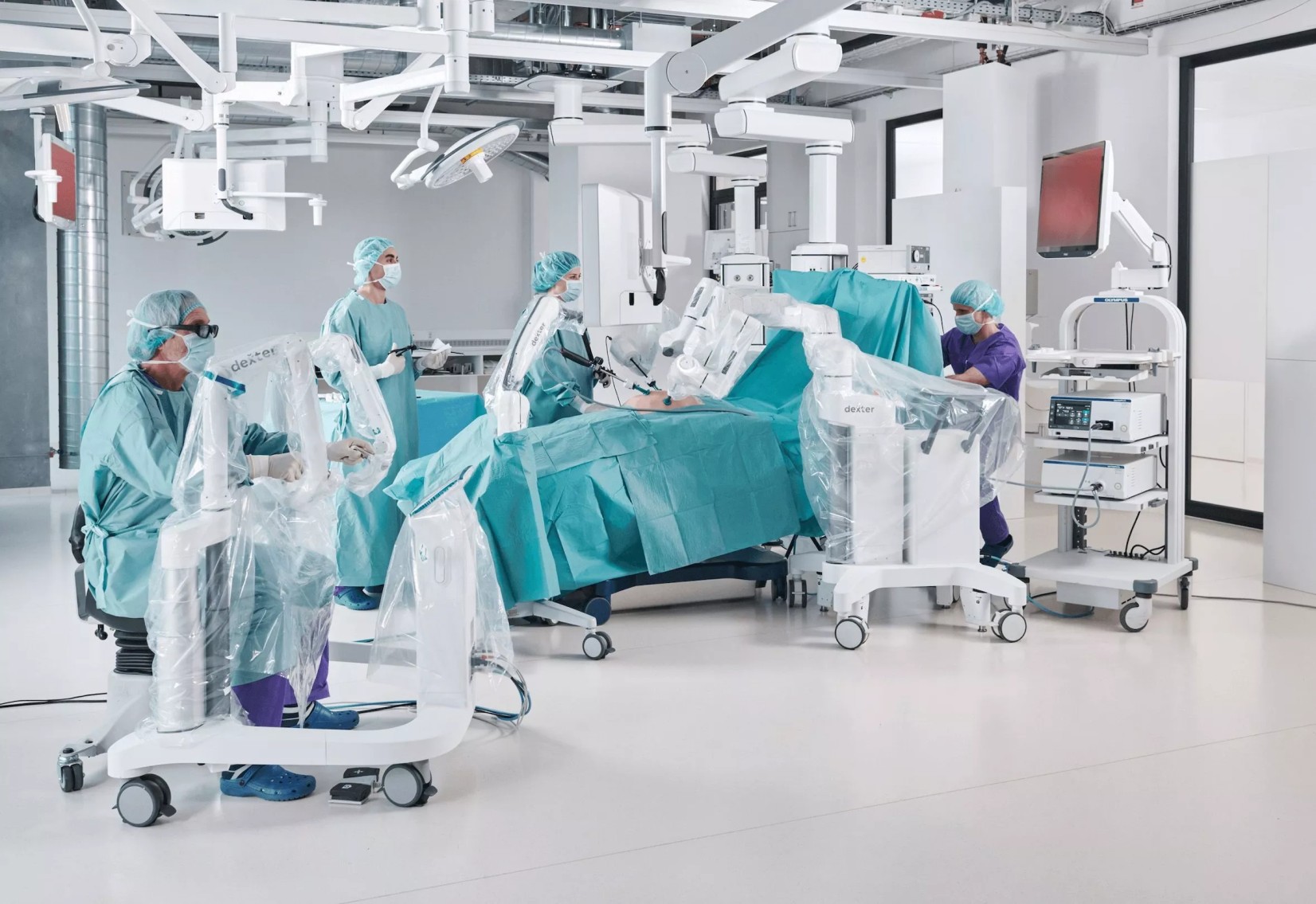
Distalmotion Accelerates U.S. Clinical Trials to Expand DEXTER® Robotic Surgery System Indications
Following FDA clearance for cholecystectomy, Distalmotion advances ventral hernia, sacrocolpopexy, and myomectomy trials as part of its strategy to broaden DEXTER’s role in high-volume outpatient procedures.
Image Courtesy: Public Domain
Distalmotion announced continued momentum in its clinical trial plans to support indication expansion of its DEXTER® Robotic Surgery System in the U.S. This announcement—focused on ventral hernia repair, sacrocolpopexy, and myomectomy clinical trials—follows recent news of the company’s completion of its hysterectomy trial and FDA clearance for use of the DEXTER system in cholecystectomy (gallbladder removal), its second U.S. indication.
As part of its regulatory pathway to broaden DEXTER use in high-volume outpatient procedures, the company is actively enrolling patients in two clinical trials:
- Ventral Hernia Trial (RAVEN): Initiated in June 2025, this general surgery study surpassed the 25% patient enrollment mark in July.
- Sacrocolpopexy Trial (SPARO): Enrollment is almost halfway complete in this gynecology study, with 42% of the target cohort enrolled.
Finally, Distalmotion announced that it has received FDA approval to initiate its pivotal trial, Dexter-Assisted Robotic Evaluation in Myomectomy (REAL-M). The study will evaluate the use of DEXTER in myomectomy procedures, with enrollment planned to begin in Q4 2025.
“The U.S. market continues to be a strategic priority for Distalmotion,” said Greg Roche, CEO of Distalmotion. “We’re pleased with the pace and execution of our ongoing trials, which are laying the groundwork for more outpatient sites to offer more patients the benefits of robotic surgery.”
Together with inguinal hernia repair and cholecystectomy, hysterectomy and ventral hernia procedures contribute the most volume to the three million total annual procedures performed in U.S. outpatient settings[1].
These developments mark continued momentum for Distalmotion’s clinical strategy and reinforce its commitment to building robust clinical evidence for the safe and effective use of DEXTER in outpatient sites of care, including ASCs.
Note: The DEXTER® Robotic Surgery System is currently authorized for use only in inguinal hernia and cholecystectomy in the United States, while in Europe, it is authorized for use in all general surgery, gynecological, and urological procedures.